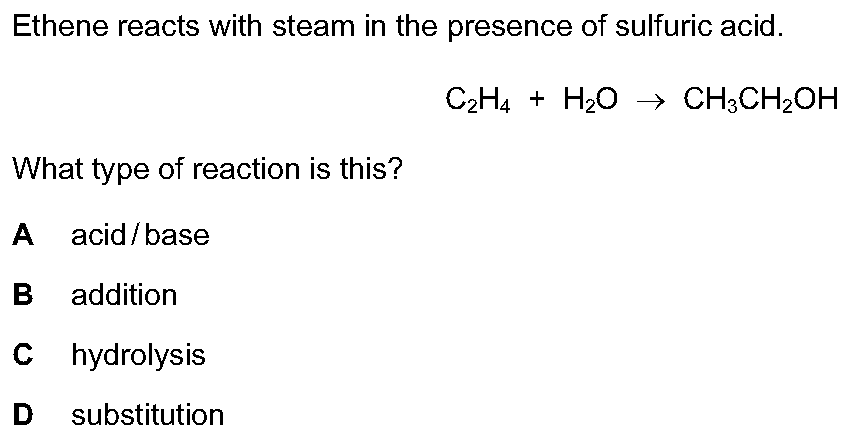
Q20:
AS & A Level Chemistry - 9701 Paper 1 2013 Summer Zone 3
Questions:
20/40

Topic: CH15 - HYDROCARBONS
Solution
Solution is B

PRACTISE
Similar Questions

LEARN
Concepts with Sparky

More Questions from this Topic
Theory
CH15 - HYDROCARBONS
Chlorine, $Cl_{2}$, reacts with many elements and compounds to form chlorides.Table 2.1 shows information about some chlorides of Period 3 elements.Ta...
2024
 Spring
Spring
 Spring
Spring
 5
5
Theory
CH15 - HYDROCARBONS
CH_3(CH_2)_5CHBrCH_3 exists as a pair of stereoisomers.(a) Draw the three-dimensional structures of the two stereoisomers of CH_3(CH_2)_5CHBrCH_3. R c...
2024
 Summer
Summer
 Summer
Summer
 2
2
Theory
CH15 - HYDROCARBONS
(a) Complete Fig. 4.2 to show the mechanism for the formation of 1,2-dibromoethane in reaction 1.Include charges, dipoles, lone pairs of electrons and...
2024
 Winter
Winter
 Winter
Winter
 2
2
Theory
CH15 - HYDROCARBONS
Hydrocarbon molecules contain covalent bonds.(a) Define covalent bond....................................................................................
2024
 Summer
Summer
 Summer
Summer
 2
2
Theory
CH15 - HYDROCARBONS
Benzene, $C_6H_6$, reacts with chloroethane, $C_2H_5Cl$, in the presence of a suitable catalyst to form ethylbenzene, $C_6H_5C_2H_5$. In the presence ...
2024
 Winter
Winter
 Winter
Winter
 2
2
Theory
CH15 - HYDROCARBONS
(a) Nitrosyl chloride, NOCl, can be formed by the reaction between nitrogen monoxide and chlorine, as shown.$2NO + Cl_{2} \rightarrow 2NOCl$The initia...
2024
 Summer
Summer
 Summer
Summer
 2
2
Theory
CH15 - HYDROCARBONS
Benzene reacts with chlorine gas to form chlorobenzene. This reaction can be described as the reaction between benzene molecules and $Cl^+$ ions. The ...
2024
 Winter
Winter
 Winter
Winter
 2
2
Theory
CH15 - HYDROCARBONS
Benzene, C_6H_6, reacts with chloroethane, C_2H_5Cl, in the presence of a suitable catalyst to form ethylbenzene, C_6H_5C_2H_5. In the presence of the...
2024
 Winter
Winter
 Winter
Winter
 2
2
Theory
CH15 - HYDROCARBONS
(a) (i) Define addition reaction.........................................................................................................................
2023
 Spring
Spring
 Spring
Spring
 2
2
Theory
CH15 - HYDROCARBONS
(a) (i) Give the systematic name of D....................................................................................................................
2023
 Winter
Winter
 Winter
Winter
 2
2
More Questions from year 2013
MCQ
CH1 - ATOMS, MOLECULES & STOICHIOMETRY
Solutions containing chlorate(I) ions are used as household bleaches and disinfectants. These solutions decompose on heating as shown.$$3 ext{ClO}^-
...
2013
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH1 - ATOMS, MOLECULES & STOICHIOMETRY
A mixture of 10 cm³ of methane and 10 cm³ of ethane was sparked with an excess of oxygen. After cooling to room temperature, the residual gas was pa...
2013
 Summer
Summer
 Summer
Summer
 1
1
MCQ
CH6 - ELECTROCHEMISTRY
The diagram shows an electrolytic cell for the extraction of aluminium. Which statement is correct?
2013
 Summer
Summer
 Summer
Summer
 1
1
MCQ
CH2 - ATOMIC STRUCTURE
Use of the Data Booklet is relevant to this question.The elements radon (Rn), francium (Fr) and radium (Ra) have proton numbers 86, 87 and 88 respecti...
2013
 Summer
Summer
 Summer
Summer
 3
3
MCQ
CH2 - ATOMIC STRUCTURE
In which species are the numbers of protons, neutrons and electrons all different?
2013
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH7 - EQUILIBRIA
An experiment is set up to measure the rate of hydrolysis of ethyl ethanoate. $$ \text{CH}_3\text{CO}_2\text{C}_2\text{H}_5 + \text{H}_2\text{O} \righ...
2013
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH5 - CHEMICAL ENERGETICS
The reaction pathway for a reversible reaction is shown below. Which statement is correct?
2013
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH8 - REACTION KINETICS
Why does the rate of a gaseous reaction increase when the pressure is increased at a constant temperature?
2013
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH4 - STATES OF MATTER
Which would behave the least like an ideal gas at room temperature?
2013
 Summer
Summer
 Summer
Summer
 1
1
MCQ
CH4 - STATES OF MATTER
The general gas equation can be used to calculate the $M_r$ value of a gas. For a sample of a gas of mass $mg$, which expression will give the value o...
2013
 Summer
Summer
 Summer
Summer
 3
3




 Share
Share




 Previous
Previous