
Q2:
AS & A Level Chemistry - 9701 Paper 3 2017 Winter Zone 1
Questions:
2/3

Topic: CH12 - AN INTRODUCTION TO THE CHEMISTRY OF TRANSITION ELEMENTS
Solution
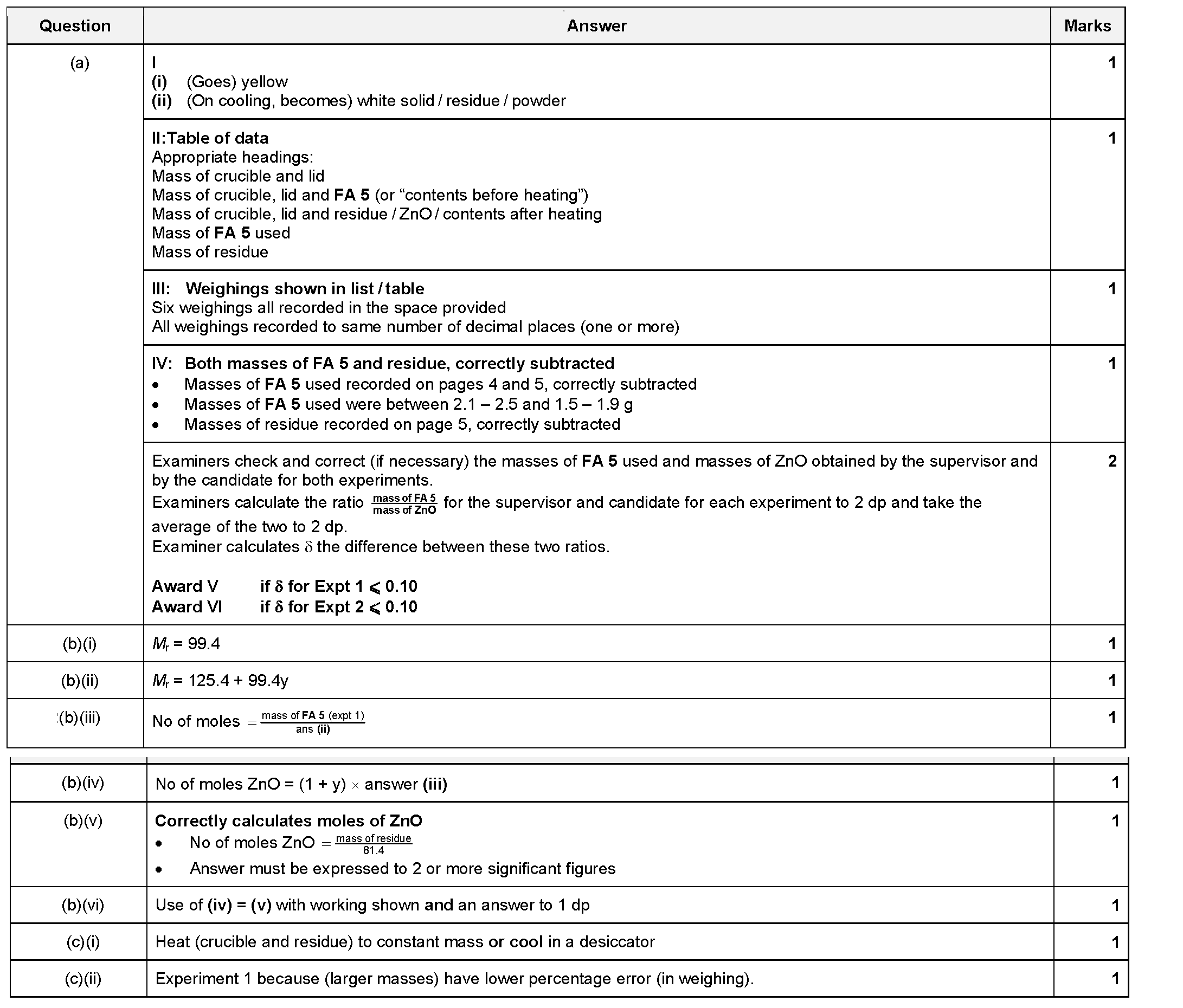


PRACTISE
Similar Questions

LEARN
Concepts with Sparky

More Questions from this Topic
Theory
CH12 - AN INTRODUCTION TO THE CHEMISTRY OF TRANSITION ELEMENTS
(a) A and B react together to give product AB.A + B → ABWhen the concentrations of A and B are both 0.0100 mol dm−3, the rate of formation of AB i...
2024
 Winter
Winter
 Winter
Winter
 3
3
Theory
CH12 - AN INTRODUCTION TO THE CHEMISTRY OF TRANSITION ELEMENTS
(a) Explain why transition elements form complex ions. .................................................................................................
2024
 Winter
Winter
 Winter
Winter
 3
3
Theory
CH12 - AN INTRODUCTION TO THE CHEMISTRY OF TRANSITION ELEMENTS
(a) (i) Define transition element........................................................................................................................
2024
 Summer
Summer
 Summer
Summer
 5
5
Theory
CH12 - AN INTRODUCTION TO THE CHEMISTRY OF TRANSITION ELEMENTS
The shapes of four different complexes, P, Q, R and S, are shown in Table 5.1. The symbol J represents an atom or ion of a transition element. The sym...
2024
 Winter
Winter
 Winter
Winter
 2
2
Theory
CH12 - AN INTRODUCTION TO THE CHEMISTRY OF TRANSITION ELEMENTS
(a) When a sample of hydrated lithium ethanedioate, $\text{Li}_2\text{C}_2\text{O}_4 \cdot \text{H}_2\text{O}$, is gently heated, two gaseous products...
2024
 Summer
Summer
 Summer
Summer
 2
2
Theory
CH12 - AN INTRODUCTION TO THE CHEMISTRY OF TRANSITION ELEMENTS
(a) (i) Explain why transition elements have variable oxidation states...................................................................................
2024
 Summer
Summer
 Summer
Summer
 3
3
Theory
CH12 - AN INTRODUCTION TO THE CHEMISTRY OF TRANSITION ELEMENTS
Iron is a transition metal in Group 8 of the Periodic Table.(a) (i) Explain why iron has variable oxidation states.......................................
2024
 Spring
Spring
 Spring
Spring
 3
3
Theory
CH12 - AN INTRODUCTION TO THE CHEMISTRY OF TRANSITION ELEMENTS
(a) The equation for reaction 1 is shown.reaction 1\qquad X \rightarrow 2YReaction 1 is first order with respect to the concentration of X. The half-l...
2024
 Winter
Winter
 Winter
Winter
 2
2
Theory
CH12 - AN INTRODUCTION TO THE CHEMISTRY OF TRANSITION ELEMENTS
Ruthenium and osmium are transition metals below iron in Group 8 of the Periodic Table.(a) Two different complex ions, $X$ and $Y$, can form when anhy...
2024
 Spring
Spring
 Spring
Spring
 2
2
Theory
CH12 - AN INTRODUCTION TO THE CHEMISTRY OF TRANSITION ELEMENTS
(a) When NaOH(aq) is added to an aqueous solution containing [Co(H2O)6]2+ a precipitation reaction occurs accompanied by a colour change.In this react...
2024
 Winter
Winter
 Winter
Winter
 4
4
More Questions from year 2017
MCQ
CH3 - CHEMICAL BONDING
Which molecule contains six bonding electrons?
2017
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH1 - ATOMS, MOLECULES & STOICHIOMETRY
The mass spectrum of a sample of lithium shows that it contains two isotopes, $^6\text{Li}$ and $^7\text{Li}$.The isotopic abundances are shown in the...
2017
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH1 - ATOMS, MOLECULES & STOICHIOMETRY
A sports medal has a total surface area of 150 cm$^2$. It was evenly coated with silver by electrolysis. Its mass increased by 0.216 g.How many atoms ...
2017
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH2 - ATOMIC STRUCTURE
Which property of an atom does not affect its first ionisation energy?
2017
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH3 - CHEMICAL BONDING
Which molecule has the largest overall dipole?
2017
 Summer
Summer
 Summer
Summer
 1
1
MCQ
CH4 - STATES OF MATTER
The complete combustion of 2 moles of a straight chain alkane produces 400 dm^3 of carbon dioxide measured at 301 K and 1 \times 10^5 Pa. Carbon dioxi...
2017
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH5 - CHEMICAL ENERGETICS
Which expression gives the standard enthalpy change of combustion of methane?
2017
 Summer
Summer
 Summer
Summer
 1
1
MCQ
CH6 - ELECTROCHEMISTRY
Solutions containing chlorate(I) ions are used as household bleaches and disinfectants. These solutions decompose on heating as shown. $$3ClO^- \righ...
2017
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH6 - ELECTROCHEMISTRY
When $K_2MnO_4$ is dissolved in water, the following reaction occurs:$aMnO_4^{2-}(aq) + bH_2O(l) \rightarrow cMnO_4^{-}(aq) + dMnO_2(s) + eOH^{-}(aq)$...
2017
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH7 - EQUILIBRIA
Methanol can be produced from hydrogen and carbon monoxide.$2H_2(g) + CO(g) \rightleftharpoons CH_3OH(g)$What is the expression for $K_p$ for this rea...
2017
 Summer
Summer
 Summer
Summer
 3
3




 Share
Share




 Previous
Previous




