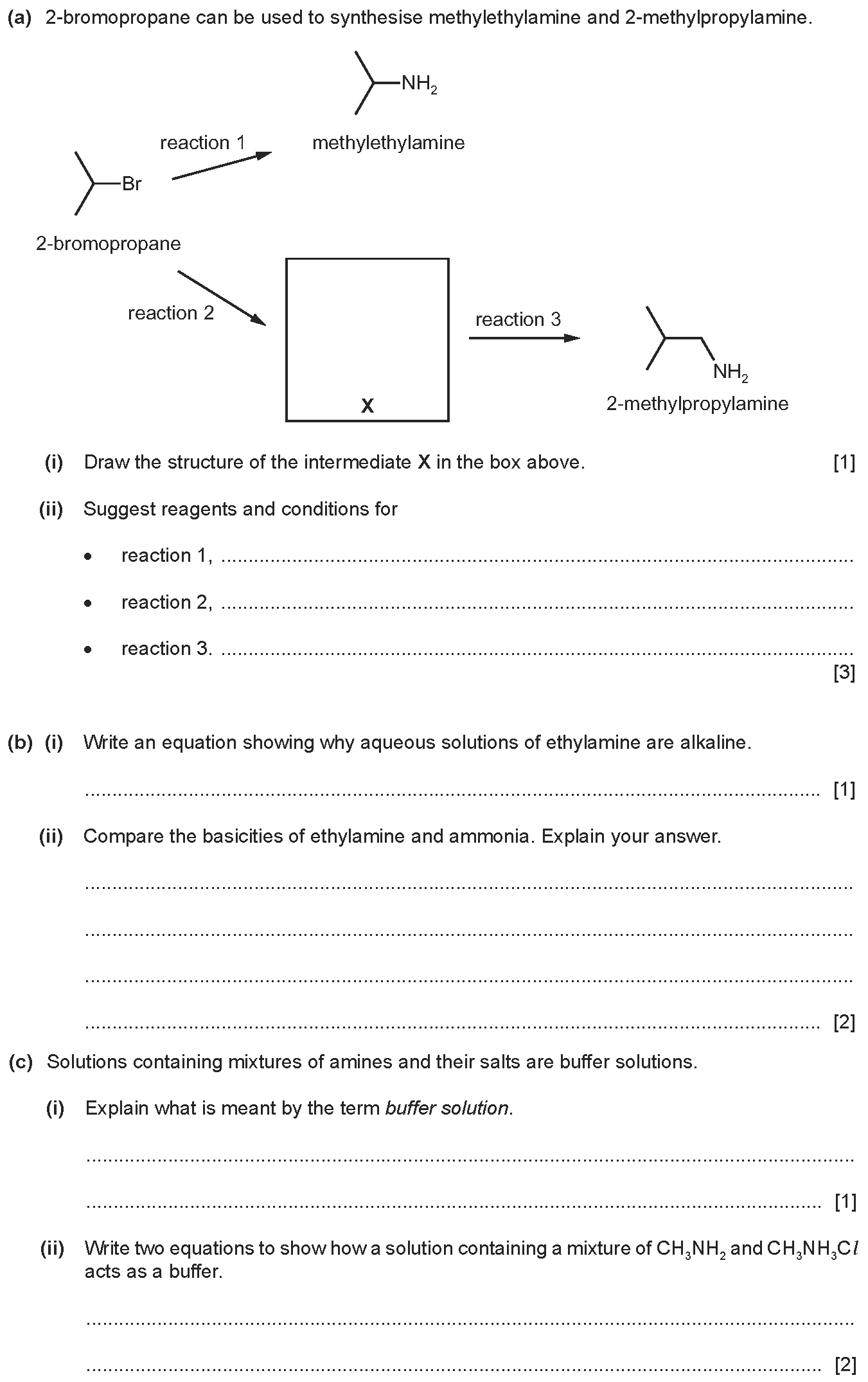
Q3:
AS & A Level Chemistry - 9701 Paper 4 2016 Summer Zone 2
Questions:
3/10

Topic: CH20 - NITROGEN COMPOUNDS
Solution


PRACTISE
Similar Questions

LEARN
Concepts with Sparky

More Questions from this Topic
Theory
CH20 - NITROGEN COMPOUNDS
Oxygen is a Group 16 element. (a) (i) Write equations for the following reactions. • sodium and oxygen • sulfur and oxygen $\text{.............
2024
 Winter
Winter
 Winter
Winter
 3
3
Theory
CH20 - NITROGEN COMPOUNDS
(a) Nitrogen is found in inorganic compounds such as nitrogen oxides (NO\(_x\)), nitrates and nitric acid.(i) Identify one natural and one man-made oc...
2024
 Winter
Winter
 Winter
Winter
 3
3
Theory
CH20 - NITROGEN COMPOUNDS
(a) \( NH_3(g) \) reacts with \( HCl(g) \) to produce \( NH_4Cl(s) \), as shown.\[ NH_3(g) + HCl(g) \rightarrow NH_4Cl(s) \]Draw a diagram to show the...
2024
 Summer
Summer
 Summer
Summer
 2
2
Theory
CH20 - NITROGEN COMPOUNDS
Nitrogen, $N_2$, is generally an unreactive molecule but it does react under certain conditions. (a) Give two reasons to explain the lack of reactivi...
2024
 Spring
Spring
 Spring
Spring
 4
4
Theory
CH20 - NITROGEN COMPOUNDS
(a) Identify compound U which contains only three elements............................................................ [1](b) Describe the reagents an...
2024
 Winter
Winter
 Winter
Winter
 2
2
Theory
CH20 - NITROGEN COMPOUNDS
(a) An aqueous solution of phenol, $C_6H_5OH$, is acidic at 298K.Explain why phenol is more acidic than water............................................
2024
 Winter
Winter
 Winter
Winter
 2
2
Theory
CH20 - NITROGEN COMPOUNDS
Amino acids are molecules that contain —NH₂ and —COOH functional groups.Glycine, $H_2NCH_2COOH$, is the simplest stable amino acid.(a) The isoel...
2024
 Spring
Spring
 Spring
Spring
 2
2
Theory
CH20 - NITROGEN COMPOUNDS
(a) State the relative basicities of phenylamine, $\text{C}_6\text{H}_5\text{NH}_2$, benzylamine, $\text{C}_6\text{H}_5\text{CH}_2\text{NH}_2$, and am...
2024
 Summer
Summer
 Summer
Summer
 1
1
Theory
CH20 - NITROGEN COMPOUNDS
(a) Isomer P and isomer Q have identical physical and chemical properties, with the exception of two specific properties. One of these two properties ...
2024
 Winter
Winter
 Winter
Winter
 3
3
Theory
CH20 - NITROGEN COMPOUNDS
A reaction scheme is shown in Fig. 7.1.The reagents needed for reaction 2 and reaction 3 are stated.Reaction 5 takes place when $C_2H_5NH_2$ is mixed ...
2024
 Winter
Winter
 Winter
Winter
 1
1
More Questions from year 2016
Theory
CH2 - ATOMIC STRUCTURE
(a) Complete the table to show the composition and identity of some atoms and ions.[Table]name of element | nucleon number | atomic number | number of...
2016
 Summer
Summer
 Summer
Summer
 1
1
Theory
CH4 - STATES OF MATTER
D, E, F, and G are four consecutive elements in the fourth period of the Periodic Table. (The letters are not the actual symbols of the elements.)D is...
2016
 Summer
Summer
 Summer
Summer
 1
1
Theory
CH10 - GROUP 2
The elements in Group 2, and their compounds, show many similarities and trends in their properties.(a) Magnesium, calcium, strontium and barium all r...
2016
 Summer
Summer
 Summer
Summer
 1
1
Theory
CH14 - AN INTRODUCTION TO ORGANIC CHEMISTRY
This question is about molecules with molecular formula $C_4H_8$.(a) Give the structures of a pair of **positional** isomers with the formula $C_4H_8$...
2016
 Summer
Summer
 Summer
Summer
 1
1
Theory
CH22 - ANALYTICAL TECHNIQUES
(a) Complete the diagram to show the mechanism of reaction 1. Include all necessary charges, partial charges, lone pairs and curly arrows.H3C—C—Br...
2016
 Summer
Summer
 Summer
Summer

Theory
CH2 - ATOMIC STRUCTURE
(a) Complete the table to show the composition and identity of some atoms and ions.[Table_1]name of element | nucleon number | atomic number | number ...
2016
 Summer
Summer
 Summer
Summer
 2
2
Theory
CH5 - CHEMICAL ENERGETICS
The elements in Group 17, the halogens, and their compounds, show many similarities and trends in their properties. Some data are given for the elemen...
2016
 Summer
Summer
 Summer
Summer
 3
3
Theory
CH6 - ELECTROCHEMISTRY
Acidified potassium dichromate(VI) can oxidise ethanedioic acid, $H_2C_2O_4$. The relevant half-equations are shown. $$Cr_2O_7^{2-} + 14H^+ + 6e^- \r...
2016
 Summer
Summer
 Summer
Summer
 2
2
Theory
CH22 - ANALYTICAL TECHNIQUES
This question is about molecules with molecular formula $C_4H_8O_2$.(a) Give the structural formulae of the pair of extbf{chain} isomers with the for...
2016
 Summer
Summer
 Summer
Summer
 2
2
Theory
CH17 - HYDROXY COMPOUNDS
A reaction sequence based on propan-1-ol is shown. (a) Reactions 1 and 2 can both be carried out using the same reagents. (i) Identify suitable rea...
2016
 Summer
Summer
 Summer
Summer
 2
2




 Share
Share




 Previous
Previous