Q3:
AS & A Level Physics - 9702 Paper 4 2014 Summer Zone 2
Questions:
3/12

Topic: CH11 - TEMPERATURE
Solution


PRACTISE
Similar Questions

LEARN
Concepts with Sparky

More Questions from this Topic
Theory
CH11 - TEMPERATURE
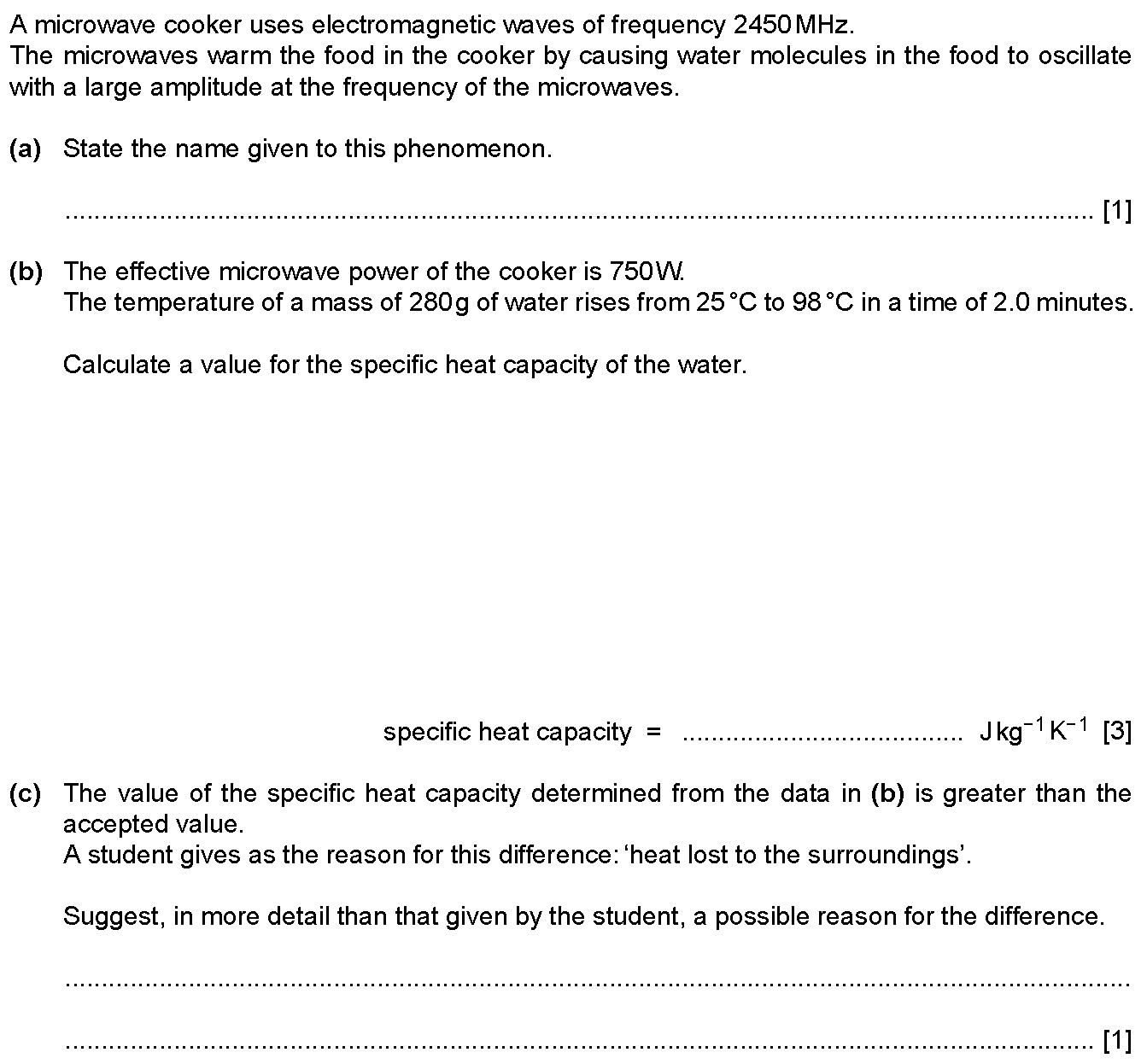
(a) State what is meant by the internal energy of a system. [2]
(b) With reference to molecular kinetic and potential energies, describe ...
2024
 Summer
Summer
 Summer
Summer
 7
7
Theory
CH11 - TEMPERATURE
(a) (i) State the magnitude and unit of absolute zero on the thermodynamic temperature scale. [1]
(ii) Explain why temperature measured u...
2024
 Summer
Summer
 Summer
Summer
 10
10
Theory
CH11 - TEMPERATURE
(a) State the first law of thermodynamics. Identify the meaning of any symbols that you use. [2]
(b) The state of an ideal gas is continu...
2023
 Summer
Summer
 Summer
Summer
 6
6
Theory
CH11 - TEMPERATURE
(a) State two of the basic assumptions of the kinetic theory of gases. [2]
(b) An ideal gas has amount of substance $n$.
The gas is initially ...
2023
 Summer
Summer
 Summer
Summer
 7
7
Theory
CH11 - TEMPERATURE
(a) State the reason why two objects that are at the same temperature are described as being in thermal equilibrium.
(b) Figure 1 shows the variation...
2023
 Summer
Summer
 Summer
Summer
 4
4
Theory
CH11 - TEMPERATURE
(a) Define specific latent heat of vaporisation. [2]
(b) The specific latent heat of vaporisation of water at atmospheric pressure of $1....
2022
 Summer
Summer
 Summer
Summer
 7
7
Theory
CH11 - TEMPERATURE
Fig. 2.1 shows a laboratory thermometer that is calibrated to measure temperature in degrees Celsius.
The thermometer makes use of the fact that th...
2022
 Winter
Winter
 Winter
Winter
 3
3
Theory
CH11 - TEMPERATURE
An ideal gas has a volume of $3.1 \times 10^{-3} \text{m}^3$ at a pressure of $8.5 \times 10^5 \text{Pa}$ and a temperature of $290\text{K}$, as shown...
2021
 Summer
Summer
 Summer
Summer
 5
5
Theory
CH11 - TEMPERATURE
(a) The first law of thermodynamics may be expressed as
$\Delta U = (+q) + (+w)$
where $\Delta U$ is the increase in internal energy of the system.
...
2020
 Winter
Winter
 Winter
Winter
 3
3
Theory
CH11 - TEMPERATURE
(a) State what is meant by the \textit{internal energy} of a system. [2]
(b) The atoms of an ideal gas occupy a container of volume $2.30...
2020
 Winter
Winter
 Winter
Winter
 3
3
More Questions from year 2014
MCQ
CH1 - PHYSICAL QUANTITIES & UNITS
Which quantity can be measured in electronvolts (eV)?
2014
 Summer
Summer
 Summer
Summer
 3
3
MCQ
CH1 - PHYSICAL QUANTITIES & UNITS
The unit of specific heat capacity is $\text{J kg}^{-1} \text{K}^{-1}$.What is its equivalent in terms of SI base units?
2014
 Summer
Summer
 Summer
Summer
 4
4
MCQ
CH1 - PHYSICAL QUANTITIES & UNITS
What is the vertical component of this displacement vector?
2014
 Summer
Summer
 Summer
Summer
 3
3
MCQ
CH2 - MEASUREMENT TECHNIQUES
The resistance of a lamp is calculated from the value of the potential difference (p.d.) across it and the value of the current passing through it.Whi...
2014
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH2 - MEASUREMENT TECHNIQUES
The display on a cathode-ray oscilloscope shows the signal produced by an electronic circuit. The time-base is set at 5.0 ns per division and the Y-ga...
2014
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH2 - MEASUREMENT TECHNIQUES
A digital caliper is used to measure the 28.50 mm width of a plastic ruler. The digital caliper reads to the nearest 0.01 mm. What is the correct way...
2014
 Summer
Summer
 Summer
Summer
 3
3
MCQ
CH3 - KINEMATICS
An experiment is performed to measure the acceleration of free fall $g$. A body falls between two fixed points. The four measurements shown below are ...
2014
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH3 - KINEMATICS
In a cathode-ray tube, an electron is accelerated uniformly in a straight line from a speed of $4 \times 10^{3} \: \text{ms}^{-1}$ to $2 \times 10^{7}...
2014
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH3 - KINEMATICS
The graph shows how the speed $v$ of a sprinter changes with time $t$ during a 100 m race.
What is the best estimate of the maximum acceleration of...
2014
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH4 - DYNAMICS
A tennis ball is dropped onto a table and bounces back up. The table exerts a force F on the ball.
Which graph best shows the variation with time t o...
2014
 Summer
Summer
 Summer
Summer
 3
3




 Share
Share




 Previous
Previous