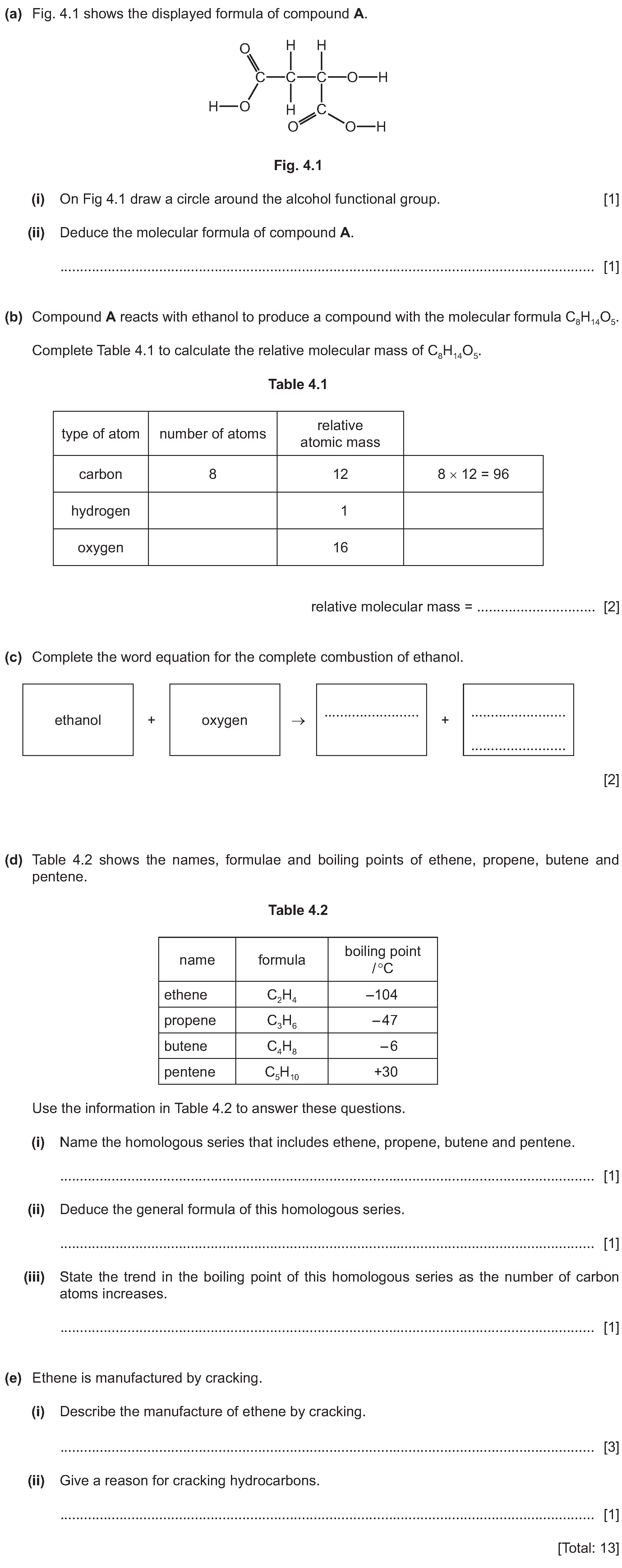
Q4:
Cambridge IGCSE Chemistry - 0620 - Core Paper 3 2024 Winter Zone 2
Questions:
4/8

Topic: Particle arrangement in solids, liquids, and gases
Solution



PRACTISE
Similar Questions

LEARN
Concepts with Sparky

More Questions from this Topic
Theory
Particle arrangement in solids, liquids, and gases
A list of substances is shown.brass, calcium oxide, carbon monoxide, diamond, glucose, hydrogen, litmus, magnesium bromide, methyl orange, sodium chlo...
2024
 Spring
Spring
 Spring
Spring
 3
3
Theory
Particle arrangement in solids, liquids, and gases
(a) Answer these questions using the information in Table 2.1 [Table_1].(i) Name the negative ion that has the highest concentration.(ii) Name the com...
2024
 Spring
Spring
 Spring
Spring
 3
3
Theory
Particle arrangement in solids, liquids, and gases
3 The chemical elements are arranged in the Periodic Table in groups and periods.(a)(i) Describe how the metallic character of the elements changes fr...
2024
 Spring
Spring
 Spring
Spring
 3
3
Theory
Particle arrangement in solids, liquids, and gases
4 Alkenes are a homologous series of hydrocarbons which are made by cracking larger alkane molecules.(a)(i) Write the general formula for alkenes.(ii)...
2024
 Spring
Spring
 Spring
Spring
 2
2
Theory
Particle arrangement in solids, liquids, and gases
5 Samarium is a metal.(a) Deduce the number of electrons and neutrons in the samarium atom shown. $^{154}_{62}\text{Sm}$Number of electrons ...Number ...
2024
 Spring
Spring
 Spring
Spring
 4
4
Theory
Particle arrangement in solids, liquids, and gases
6 Sulfur is an element in Group VI of the Periodic Table.(a) State the meaning of the term element.(b) Sulfur has a relative atomic mass of 32. Comple...
2024
 Spring
Spring
 Spring
Spring
 4
4
Theory
Particle arrangement in solids, liquids, and gases
7 Magnesium is an element in Group II of the Periodic Table.(a) Deduce the electronic configuration of magnesium.(b) Magnesium can be produced by redu...
2024
 Spring
Spring
 Spring
Spring
 3
3
Theory
Particle arrangement in solids, liquids, and gases
Fig. 1.1 shows the structures of seven substances, A, B, C, D, E, F and G. (a) Answer the following questions using only the structures in Fig. 1.1. E...
2024
 Summer
Summer
 Summer
Summer
 3
3
Theory
Particle arrangement in solids, liquids, and gases
(a) Table 2.1 shows the percentages by mass of the elements present in the human body. [Table_1]Answer these questions using information from Table 2....
2024
 Summer
Summer
 Summer
Summer
 2
2
Theory
Particle arrangement in solids, liquids, and gases
3 Aluminium is extracted by electrolysis of its purified ore.(a) Name the main ore of aluminium. [1](b) Fig. 3.1 shows the apparatus used in the extra...
2024
 Summer
Summer
 Summer
Summer
 3
3
More Questions from year 2024
MCQ
Properties of solids, liquids, and gases
Which statement about a solid, a liquid or a gas is correct?
2024
 Spring
Spring
 Spring
Spring
 6
6
MCQ
Properties of solids, liquids, and gases
Which diagram represents a mixture of compounds?
2024
 Spring
Spring
 Spring
Spring
 3
3
MCQ
Properties of solids, liquids, and gases
Four ions are listed. $$\text{N}^{3-}\quad \text{Al}^{3+}\quad \text{Li}^{+}\quad \text{Cl}^{-}$$ Which pair of ions have the same electronic configur...
2024
 Spring
Spring
 Spring
Spring
 6
6
MCQ
Properties of solids, liquids, and gases
Which statement about isotopes is correct?
2024
 Spring
Spring
 Spring
Spring
 2
2
MCQ
Properties of solids, liquids, and gases
Which statement about the ions formed by the elements in Group VII of the Periodic Table is correct?
2024
 Spring
Spring
 Spring
Spring
 2
2
MCQ
Properties of solids, liquids, and gases
Which row describes the properties of potassium bromide? [Table_1]
2024
 Spring
Spring
 Spring
Spring
 2
2
MCQ
Properties of solids, liquids, and gases
Which statement explains why graphite is used as a lubricant?
2024
 Spring
Spring
 Spring
Spring
 2
2
MCQ
Properties of solids, liquids, and gases
What is the balanced equation for the reaction between magnesium and dilute sulfuric acid?
2024
 Spring
Spring
 Spring
Spring
 3
3
MCQ
Properties of solids, liquids, and gases
The relative atomic mass, $A_r$, of an element is the average mass of the isotopes of that element compared to another particle. Which particle is us...
2024
 Spring
Spring
 Spring
Spring
 2
2
MCQ
Properties of solids, liquids, and gases
The equations for two reactions are shown.equation 1\ C + CO_2 \rightarrow x\ COequation 2\ 2C_2H_6 + 7O_2 \rightarrow 4CO_2 + y\ H_2OWhich row shows ...
2024
 Spring
Spring
 Spring
Spring
 2
2




 Share
Share




 Previous
Previous