Q29:
Cambridge IGCSE Chemistry - 0620 - Core Paper 1 2009 Summer Zone 1
Questions:
29/40

Topic: CH13 - THE BEHAVIOR OF METALS
Solution
Solution is C

PRACTISE
Similar Questions

LEARN
Concepts with Sparky

More Questions from this Topic
MCQ
CH13 - THE BEHAVIOR OF METALS
What is the balanced chemical equation for the reaction between calcium and water?
2013
 Summer
Summer
 Summer
Summer
 1
1
MCQ
CH13 - THE BEHAVIOR OF METALS
A student carried out an experiment to find the order of reactivity of five metals.They were tested with cold water, hot water and steam and the resul...
2014
 Summer
Summer
 Summer
Summer
 3
3
MCQ
CH13 - THE BEHAVIOR OF METALS
A metal has the following properties:• It does not react with cold water.• It reacts with dilute hydrochloric acid.• It cannot be extracted from...
2012
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH13 - THE BEHAVIOR OF METALS
Which statement is correct for the element of proton number 19?
2011
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH13 - THE BEHAVIOR OF METALS
A student added dilute hydrochloric acid to four metals and recorded the results. Not all of the results are correct. [Table_1] Which two results ar...
2010
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH13 - THE BEHAVIOR OF METALS
Which statements about the general properties of metals are correct?1. conduct electricity when solid2. form acidic oxides3. high melting point
2012
 Summer
Summer
 Summer
Summer
 3
3
MCQ
CH13 - THE BEHAVIOR OF METALS
A substance, X, has the following properties.1 It has a high melting point.2 It conducts electricity in the solid and liquid states.3 It is malleable....
2013
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH13 - THE BEHAVIOR OF METALS
The table shows the results of adding three metals, P, Q and R, to dilute hydrochloric acid and to water.[Table_1]What is the order of reactivity of t...
2011
 Summer
Summer
 Summer
Summer
 3
3
MCQ
CH13 - THE BEHAVIOR OF METALS
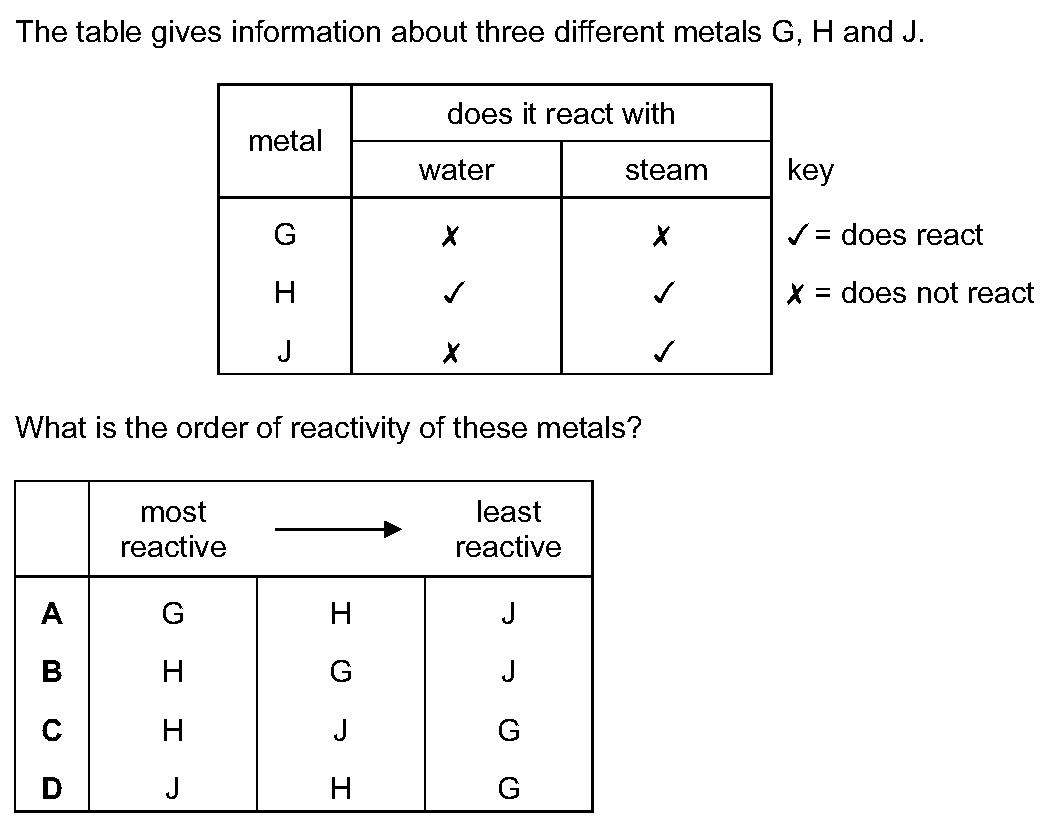
The table gives information about three different metals G, H and J.[Table_1]What is the order of reactivity of these metals?
2009
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH13 - THE BEHAVIOR OF METALS
Which substance is a metal?[Table_1]
2011
 Summer
Summer
 Summer
Summer
 2
2
More Questions from year 2009
MCQ
CH1 - STATES OF MATTER
The diagram shows how the molecules in the exhaust gases diffuse into the air. Which statement describes what happens to these molecules next?
2009
 Summer
Summer
 Summer
Summer
 8
8
MCQ
CH2 - SEPARATING SUBSTANCES
A student takes 2 g samples of calcium carbonate and adds them to 20 cm^3 samples of dilute hydrochloric acid at different temperatures. She measures ...
2009
 Summer
Summer
 Summer
Summer
 1
1
MCQ
CH2 - SEPARATING SUBSTANCES
The diagram shows the paper chromatograms of four substances, W, X, Y and Z.Which two substances are pure?
2009
 Summer
Summer
 Summer
Summer
 5
5
MCQ
CH3 - ATOMS AND ELEMENTS
An element S has the proton number 18. The next element in the Periodic Table is an element T. Which statement is correct?
2009
 Summer
Summer
 Summer
Summer
 3
3
MCQ
CH3 - ATOMS AND ELEMENTS
Which numbers are added together to give the nucleon number of an ion?
2009
 Summer
Summer
 Summer
Summer
 1
1
MCQ
CH3 - ATOMS AND ELEMENTS
The electronic configuration of an ion is 2.8.8. What could this ion be?[Table showing options for $S^{2-}$ and $Ca^{2+}$]
2009
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH4 - ATOMS COMBINING
The diagrams show the structures of two forms, P and Q, of a solid element. What are suitable uses of P and Q, based on their structures?
2009
 Summer
Summer
 Summer
Summer
 4
4
MCQ
CH4 - ATOMS COMBINING
Element V forms an acidic, covalent oxide. Which row in the table shows how many electrons there could be in the outer shell of an atom of V? [Table_1...
2009
 Summer
Summer
 Summer
Summer
 5
5
MCQ
CH4 - ATOMS COMBINING
When sodium chloride is formed from its elements, each chlorine atom ...... 1 ...... one ...... 2 .......Which words correctly complete gaps 1 and 2?
2009
 Summer
Summer
 Summer
Summer
 5
5
MCQ
CH5 - REACTING MASSES AND CHEMICAL EQUATIONS
Nitrogen and hydrogen react together to form ammonia.$N_2 + 3H_2 \rightarrow 2NH_3$When completely converted, 7 tonnes of nitrogen gives 8.5 tonnes of...
2009
 Summer
Summer
 Summer
Summer
 1
1




 Share
Share




 Previous
Previous