Q23:
Cambridge IGCSE Chemistry - 0620 - Core Paper 1 2014 Summer Zone 2
Questions:
23/40

Topic: CH13 - THE BEHAVIOR OF METALS
Solution
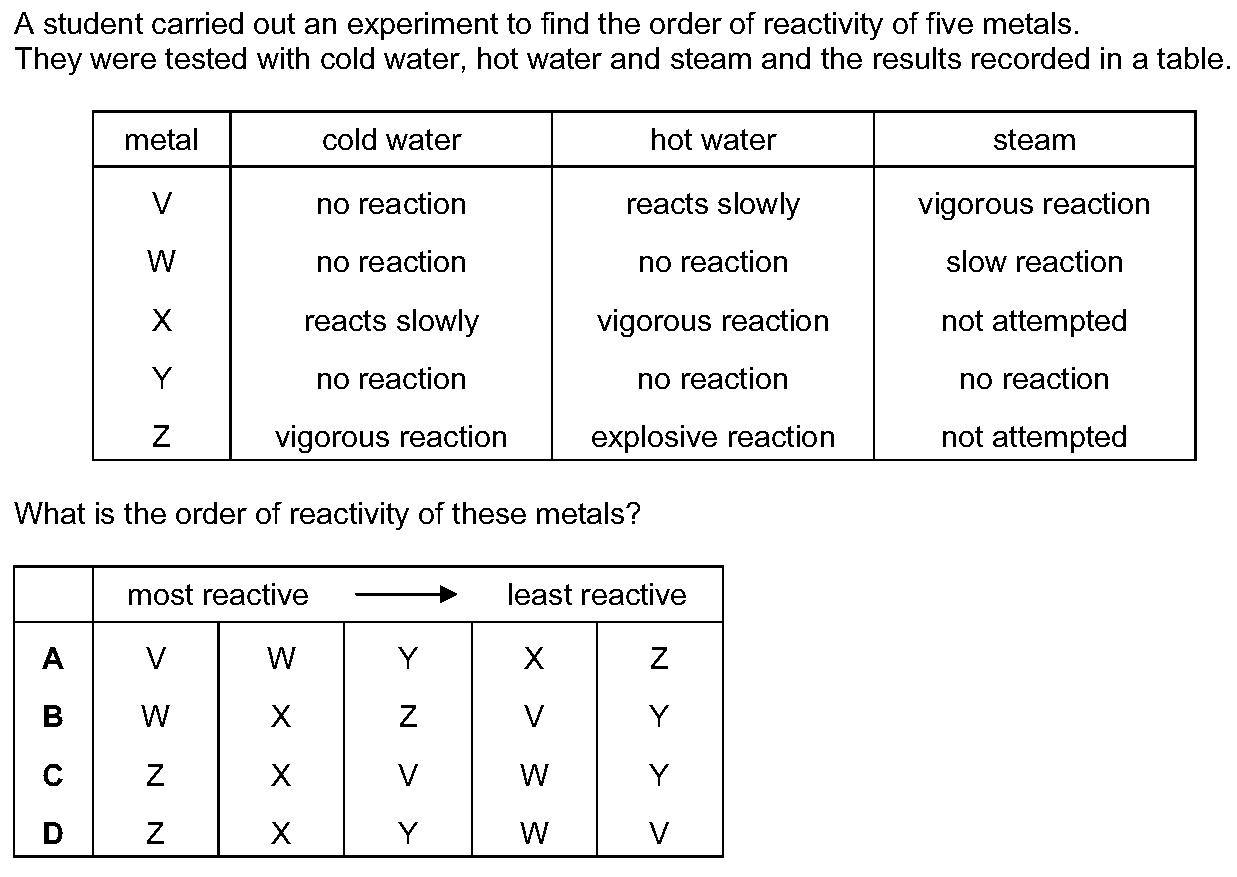
Solution is C

PRACTISE
Similar Questions

LEARN
Concepts with Sparky

More Questions from this Topic
MCQ
CH13 - THE BEHAVIOR OF METALS
What is the balanced chemical equation for the reaction between calcium and water?
2013
 Summer
Summer
 Summer
Summer
 1
1
MCQ
CH13 - THE BEHAVIOR OF METALS
A student carried out an experiment to find the order of reactivity of five metals.They were tested with cold water, hot water and steam and the resul...
2014
 Summer
Summer
 Summer
Summer
 3
3
MCQ
CH13 - THE BEHAVIOR OF METALS
A metal has the following properties:• It does not react with cold water.• It reacts with dilute hydrochloric acid.• It cannot be extracted from...
2012
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH13 - THE BEHAVIOR OF METALS
Which statement is correct for the element of proton number 19?
2011
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH13 - THE BEHAVIOR OF METALS
A student added dilute hydrochloric acid to four metals and recorded the results. Not all of the results are correct. [Table_1] Which two results ar...
2010
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH13 - THE BEHAVIOR OF METALS
Which statements about the general properties of metals are correct?1. conduct electricity when solid2. form acidic oxides3. high melting point
2012
 Summer
Summer
 Summer
Summer
 3
3
MCQ
CH13 - THE BEHAVIOR OF METALS
A substance, X, has the following properties.1 It has a high melting point.2 It conducts electricity in the solid and liquid states.3 It is malleable....
2013
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH13 - THE BEHAVIOR OF METALS
The table shows the results of adding three metals, P, Q and R, to dilute hydrochloric acid and to water.[Table_1]What is the order of reactivity of t...
2011
 Summer
Summer
 Summer
Summer
 3
3
MCQ
CH13 - THE BEHAVIOR OF METALS
The table gives information about three different metals G, H and J.[Table_1]What is the order of reactivity of these metals?
2009
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH13 - THE BEHAVIOR OF METALS
Which substance is a metal?[Table_1]
2011
 Summer
Summer
 Summer
Summer
 2
2
More Questions from year 2014
MCQ
CH1 - STATES OF MATTER
The diagram shows the result of dropping a purple crystal into water. Which processes take place in this experiment?
2014
 Summer
Summer
 Summer
Summer
 1
1
MCQ
CH2 - SEPARATING SUBSTANCES
The four pieces of apparatus shown below are used in chemical experiments. Which statement about the apparatus is correct?
2014
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH2 - SEPARATING SUBSTANCES
Alcohol and water are completely miscible. This means when mixed together they form only one liquid layer. Which method is used to separate alcohol fr...
2014
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH3 - ATOMS AND ELEMENTS
The diagram shows the structure of an atom of element X. What is X?
2014
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH3 - ATOMS AND ELEMENTS
The diagrams show four particles.Which two diagrams show atoms that are isotopes of each other?
2014
 Summer
Summer
 Summer
Summer
 3
3
MCQ
CH4 - ATOMS COMBINING
The 'lead' in a pencil is made of a mixture of graphite and clay. When the percentage of graphite is increased, the pencil slides across the paper mo...
2014
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH4 - ATOMS COMBINING
Element X is in Group I of the Periodic Table. X reacts with element Y to form an ionic compound.Which equation shows the process that takes place whe...
2014
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH4 - ATOMS COMBINING
Solid F is an element. Solid G is a compound. Neither solid conducts electricity but G conducts electricity when dissolved in water. These properties ...
2014
 Summer
Summer
 Summer
Summer
 1
1
MCQ
CH5 - REACTING MASSES AND CHEMICAL EQUATIONS
A compound contains one atom of calcium, two atoms of hydrogen and two atoms of oxygen. What is the correct chemical formula of the compound?
2014
 Summer
Summer
 Summer
Summer
 2
2
MCQ
CH5 - REACTING MASSES AND CHEMICAL EQUATIONS
In athletics, banned drugs such as nandrolone have been taken illegally to improve performance. Nandrolone has the molecular formula $C_{18}H_{26}O_2$...
2014
 Summer
Summer
 Summer
Summer
 1
1




 Share
Share




 Previous
Previous